Abstract
Background
AFM13 is a bispecific, tetravalent NK cell-engaging antibody construct binding to CD30 on Hodgkin Lymphoma (HL) cells and CD16A on NK cells. By engaging CD16A-positive NK cells, AFM13 leads to NK-cell mediated killing of CD30-positive lymphoma cells (Reusch et al., 2014). Pembrolizumab is a PD-1 blocking antibody that prevents tumor immune evasion and has shown to induce high single-agent response rates in patients (pts) with relapsed or refractory (R/R) HL (Armand et al., 2016). AFM13 has shown first signs of clinical activity in R/R HL as single agent in a preceding Phase 1 study (Rothe et al., 2015). Preclinical in vivo data of the combination of AFM13 with PD-1 inhibition suggest synergistic activity and the potential for induction of cross-talk between innate and adaptive immunity (Zhao et al., 2016). The combination of the two agents could improve outcomes in pts with R/R HL.
Methods
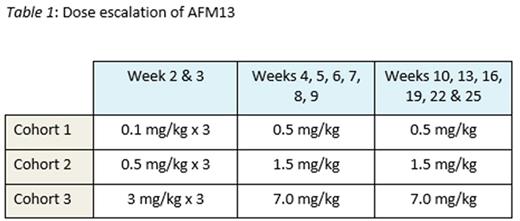
A Phase 1b study is ongoing to evaluate the safety and tolerability of the combination of AFM13 with pembrolizumab (Keytruda) as salvage therapy after failure of standard therapies including brentuximab vedotin in HL (NCT02665650). Pts receive escalating doses of AFM13 (Table 1) in combination with pembrolizumab at a flat dose of 200 mg administered every 3 weeks following the classical 3+3 design. Upon completion, recruitment continues into an extension cohort. Response assessment is performed every 12 weeks by PET/CT according to the Lugano Classification Revised Staging System (Cheson 2014).
Results
As of July 24th 2017, 12 pts have been enrolled into the dose escalation part of the study. All pts have relapsed disease and have failed standard treatments including brentuximab vedotin. 66 % (8/12) have failed prior autologous stem cell transplantation.
At the time of the data extract, all 12 pts had completed the 6-week dose-limiting toxicity (DLT) observation period. 3 pts were enrolled into cohorts 1 and 2 each and 6 patients were enrolled into cohort 3. While no grade 3 or 4 adverse events related to the study treatment were observed, one DLT per protocol definition was observed in cohort 3, which was a repeated grade 2 infusion-related reaction (IRR), leading to discontinuation of AFM13 treatment. No further DLTs occurred. The most frequent adverse events (AEs) were IRRs (9 pts), headache (4 pts), rash (4 pts), nausea (4 pts) and cough (4 pts). All these events were of grade 1 or 2. All observed IRRs were related to the AFM13 administration. Premedication with antihistamine and steroid was not mandatory during dose escalation. Only one grade 3 AE, a duodenal ulcer, was observed, which was assessed as not related to the study treatment. Thus the extension cohort will continue with the highest dose explored during dose escalation.
3-month response data from the 12 patients enrolled into cohorts 1, 2 and 3 will be reported at the ASH 2017 annual meeting.
Conclusions
Early data suggest that the combination of AFM13 and pembrolizumab is a well-tolerated salvage therapy in pts with R/R HL. While IRRs were observed frequently, all of these events were of mild or moderate severity and only one event led to discontinuation of treatment in one patient. Preliminary antitumor activity will be further analyzed in the currently recruiting extension cohort.
Ansell: Merck: Research Funding; Bristol-Myers Squibb: Research Funding; Celldex: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding. Chen: Merck: Consultancy, Speakers Bureau; Genentech: Speakers Bureau; Seattle Genetics: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Affimed: Research Funding; Pharmacyclics: Consultancy, Research Funding. Forero-Torres: Juno: Research Funding; 47 Pharmaceutical: Research Funding; Affimed: Research Funding; Pfizer: Research Funding; Seattle Genetics: Consultancy, Research Funding, Speakers Bureau; Genentech/Roche: Research Funding; GSK: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Armand: Tensha: Research Funding; Affimed: Research Funding; Roche: Research Funding; Otsuka: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Infinity: Consultancy; Merck & Co., Inc.: Consultancy, Research Funding; Genmab: Consultancy; Sequenta/Adaptive: Research Funding; Sigma Tau: Research Funding. Lossos: Affimed: Research Funding. Reeder: Novartis: Research Funding; Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; Millennium: Research Funding; Affimed: Research Funding. Strassz: Affimed: Employment. Kerber: Affimed: Employment. Bartlett: Celgene: Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck & Co: Research Funding; Bristol-Meyers Squibb: Research Funding; Immune Design: Research Funding; Forty Seven: Research Funding; Affimed: Research Funding; Janssen: Research Funding; Pharmacyclics: Research Funding; Millenium: Research Funding; Astra Zeneca: Research Funding; ImaginAB: Research Funding; Novartis: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal